A great number of other brands with fancy names have gone out of the German market, because of some defects in the processes of manufacture. The English exporters, as a rule, offer three or four grades of lithopone, the lowest priced consisting of about 12 per cent zinc sulphide, the best varying between 30 and 32 per cent zinc sulphide. A white pigment of this composition containing more than 32 per cent zinc sulphide does not work well in oil as a paint, although in the oilcloth and shade cloth industries an article containing as high as 45 per cent zinc sulphide has been used apparently with success. Carefully prepared lithopone, containing 30 to 32 per cent sulphide of zinc with not over 1.5 per cent zinc oxide, the balance being barium sulphate, is a white powder almost equal to the best grades of French process zinc oxide in whiteness and holds a medium position in specific gravity between white lead and zinc oxide. Its oil absorption is also fairly well in the middle between the two white pigments mentioned, lead carbonate requiring 9 per cent of oil, zinc oxide on an average 17 per cent and lithopone 13 per cent to form a stiff paste. There is one advantage in the manipulation of lithopone in oil over both white lead and zinc oxide, it is more readily mis-cible than either of these, for some purposes requiring no mill grinding at all, simply thorough mixing with the oil. However, when lithopone has not been furnaced up to the required time, it will require a much greater percentage of oil for grinding and more thinners for spreading than the normal pigment. Pigment of that character is not well adapted for use in the manufacture of paints, as it lacks in body and color resisting properties and does not work well under the brush. In those industries, where the paint can be applied with machinery, as in shade cloth making, etc., it appears to be preferred, because of these very defects. As this sort of lithopone, ground in linseed oil in paste form, is thinned for application to the cloth with benzine only, and on account of its greater tendency to thicken, requires more of this comparatively cheap thinning medium, it is preferred by most of the manufacturers of machine painted shade cloth. Another point considered by them is that it does not require as much coloring matter to tint the white paste to the required standard depth as would be the case if the lithopone were of the standard required for the making of paint or enamels. On the other hand, the lithopone preferred by the shade cloth trade would prove a failure in the manufacture of oil paints and much more so, when used as a pigment in the so-called enamel or varnish paints. Every paint manufacturer knows, or should know, that a pigment containing hygroscopic moisture does not work well with oil and driers in a paint and that with varnish especially it is very susceptible to livering on standing and to becoming puffed to such an extent as to make it unworkable under the brush. While the process of making lithopone is not very difficult or complicated, the success of obtaining a first class product depends to a great extent on the purity of the material used. Foreign substances in these are readily eliminated by careful manipulation, which, however, requires thorough knowledge and great care, as otherwise the result will be a failure, rendering a product of bad color and lack of covering power.

 Alternatively, they can complement equally vibrant clothing for a bold, statement-making look Alternatively, they can complement equally vibrant clothing for a bold, statement-making look
Alternatively, they can complement equally vibrant clothing for a bold, statement-making look Alternatively, they can complement equally vibrant clothing for a bold, statement-making look
 By working collaboratively with suppliers to identify new opportunities, source high-quality products at affordable prices, and resolve any issues that arise quickly and effectively, businesses can enjoy a steady flow of high-quality inventory and reduce costs associated with supply chain disruptions By working collaboratively with suppliers to identify new opportunities, source high-quality products at affordable prices, and resolve any issues that arise quickly and effectively, businesses can enjoy a steady flow of high-quality inventory and reduce costs associated with supply chain disruptions
By working collaboratively with suppliers to identify new opportunities, source high-quality products at affordable prices, and resolve any issues that arise quickly and effectively, businesses can enjoy a steady flow of high-quality inventory and reduce costs associated with supply chain disruptions By working collaboratively with suppliers to identify new opportunities, source high-quality products at affordable prices, and resolve any issues that arise quickly and effectively, businesses can enjoy a steady flow of high-quality inventory and reduce costs associated with supply chain disruptions


 Hunting environments can be damp and rugged, so having a shoe that shields against moisture and holds up against jagged edges is paramount Hunting environments can be damp and rugged, so having a shoe that shields against moisture and holds up against jagged edges is paramount
Hunting environments can be damp and rugged, so having a shoe that shields against moisture and holds up against jagged edges is paramount Hunting environments can be damp and rugged, so having a shoe that shields against moisture and holds up against jagged edges is paramount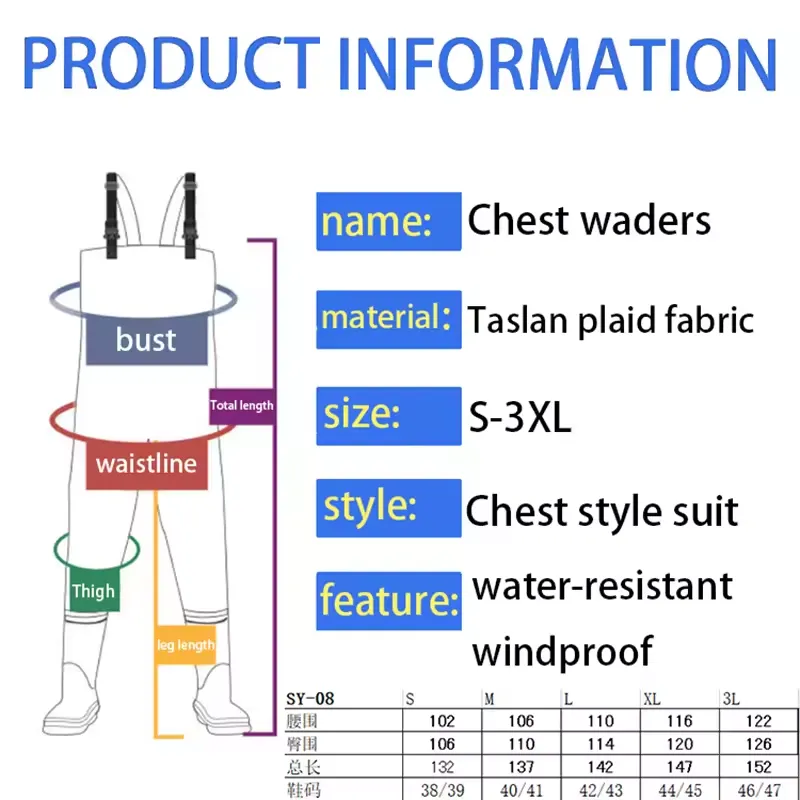 Moreover, rubber is known for its robustness and grip, which means these boots can handle both urban sidewalks and more rugged terrains with equal aplomb Moreover, rubber is known for its robustness and grip, which means these boots can handle both urban sidewalks and more rugged terrains with equal aplomb
Moreover, rubber is known for its robustness and grip, which means these boots can handle both urban sidewalks and more rugged terrains with equal aplomb Moreover, rubber is known for its robustness and grip, which means these boots can handle both urban sidewalks and more rugged terrains with equal aplomb
 This can help ensure a steady supply of raw materials and reduce transportation costs, ultimately allowing suppliers to offer more competitive prices to their customers This can help ensure a steady supply of raw materials and reduce transportation costs, ultimately allowing suppliers to offer more competitive prices to their customers
This can help ensure a steady supply of raw materials and reduce transportation costs, ultimately allowing suppliers to offer more competitive prices to their customers This can help ensure a steady supply of raw materials and reduce transportation costs, ultimately allowing suppliers to offer more competitive prices to their customers However, the specific attributes of anatase make it irreplaceable in certain sectors However, the specific attributes of anatase make it irreplaceable in certain sectors
However, the specific attributes of anatase make it irreplaceable in certain sectors However, the specific attributes of anatase make it irreplaceable in certain sectors

 It is generally more expensive than lithopone and may not be as environmentally friendly It is generally more expensive than lithopone and may not be as environmentally friendly
It is generally more expensive than lithopone and may not be as environmentally friendly It is generally more expensive than lithopone and may not be as environmentally friendly